
[ad_1]
While it may be the reason behind tires skidding, pipes bursting, and closed roads making traffic a nightmare, ice doesn’t always form as easily as it seems. It often gets an assist from proteins made by fungi.
Never mind the common thinking that ice forms at 0° C (32° F). Though this is water’s freezing point, pure water will only freeze when temperatures plummet as low as minus 46° C (minus 50.8° F). So why does it usually freeze at zero anyway? Organisms such as bacteria, insects, and fungi produce proteins known as ice nucleators (non-protein nucleators can also be of abiotic origin). These proteins can kick-start the formation, or nucleation, of ice at higher temperatures than pure water would freeze at.
While the exact reason fungi make these proteins remains unknown, researchers Valeria Molinero of the University of Utah and Konrad Meister of Boise State University led a study that has revealed more about how fungal ice nucleators can both promote and hold back ice formation more efficiently than those of many other life-forms.
Getting to the nucleus
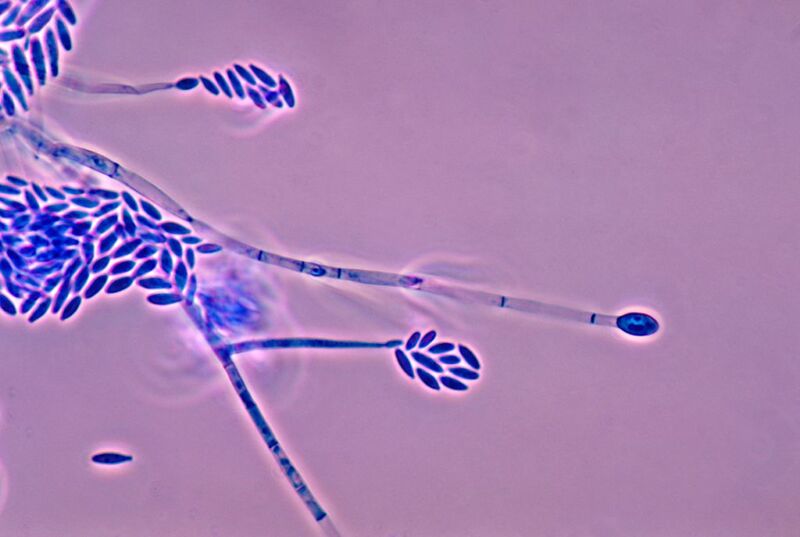
Organisms capable of producing ice nucleators belong to different biological kingdoms but are thought to have evolved the same ability independently—a phenomenon known as convergent evolution. Fungal ice nucleators had been something of an enigma until Molinero, Meister, and their team studied the nucleators produced by the fungus Fusarium acuminatum.
“We find ice-binding and ice-shaping activity of Fusarium [ice nucleators], suggesting a potential connection between ice growth and inhibition,” the scientists said in a study recently published in PNAS.
Ice nucleators help freeze water by fast-forwarding nucleation, which is the process by which water molecules initiate the formation of ice crystals. Forming a crystal means molecules of H2O must align themselves in specific orientations to form a rigid lattice structure. This starts as a small group of latticed molecules, a “nucleus” in liquid water. If this nucleus is large enough, more water molecules will aggregate on it until an ice crystal forms.
This is difficult for pure water to do on its own because the motion of its molecules makes it difficult to form a nucleus. Ice nucleator proteins help water molecules aggregate into a nucleus. Some bacterial nucleators are so effective that the bacteria that produce them are used to make snow at ski resorts.
Frozen in mystery
Fusarium nucleators are also highly efficient and effective. Meister and Molinero’s team found that these fungal proteins are much smaller than many of those synthesized by bacteria and other organisms, and large numbers of them join together into complex structures that help ice nucleate. Even when the levels of Fusarium proteins were reduced in the lab, they could still trigger the nucleation process.
The scientists have a hypothesis as to why Fusarium and some other organisms evolved such small molecules that aggregate so efficiently: “We expect that the energetic benefit for the organism in producing smaller proteins, rather than a single large one, contributes to the success and adoption of [this] strategy across species that are not evolutionary-related,” they said in the same study.
Another question—one where the answer has not crystallized yet—is why fungi use ice nucleators. Whether they are meant to promote ice formation or whether it is an incidental side effect of another benefit they provide to the fungus still needs to be researched.
But creatures like frogs might give us a clue. Many frog species have a natural antifreeze that keeps them alive while they fall into a state of torpor during the colder months. It is not uncommon for them to end up covered in ice, but ice nucleators produced in their cells help them control where ice forms, keeping them alive (along with an antifreeze made of urea and glucose). Some species also ingest bacteria that help with the process. Could ice nucleators in fungi be part of a similar antifreeze system? At this point, nobody knows.
As more continues to be demystified about biological and abiotic ice nucleators, they could eventually be used for more efficient methods of freezing food, making snow, creating clouds, and possibly the cryogenic freezing of human cells, which was recently successful. The future is frozen.
PNAS, 2023. DOI: 10.1073/pnas.2303243120
[ad_2]